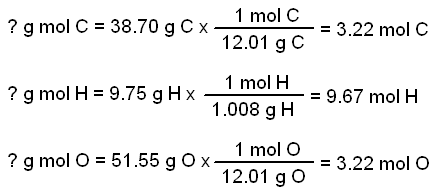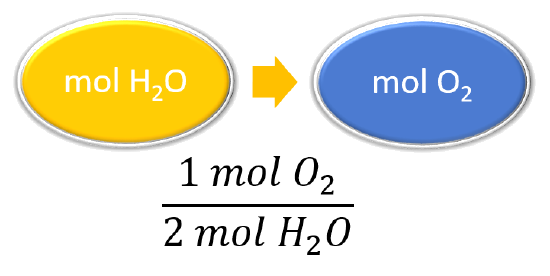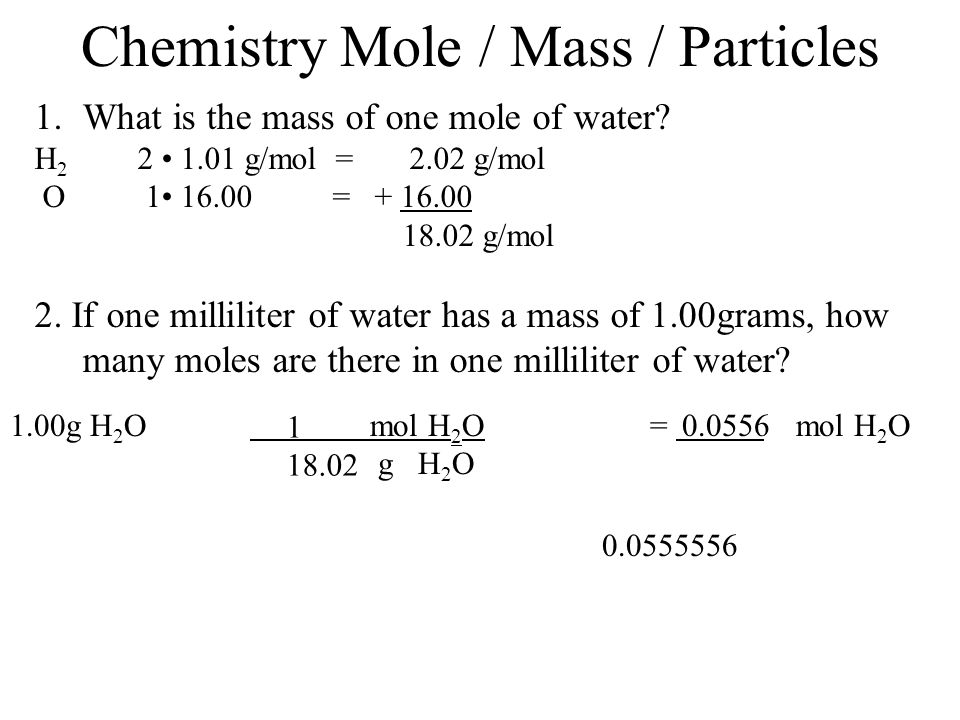
Chemistry Warm Up: Mole / Mass / Particles 1.What is the mass of one mole of water? 2.If one milliliter of water has a mass of 1.00grams, how many moles. - ppt download

Mole Concept and Chemical Calculations: Difference between Relative Atomic Mass, Relative Molecular Mass, Relative Formula Mass and Molar Mass
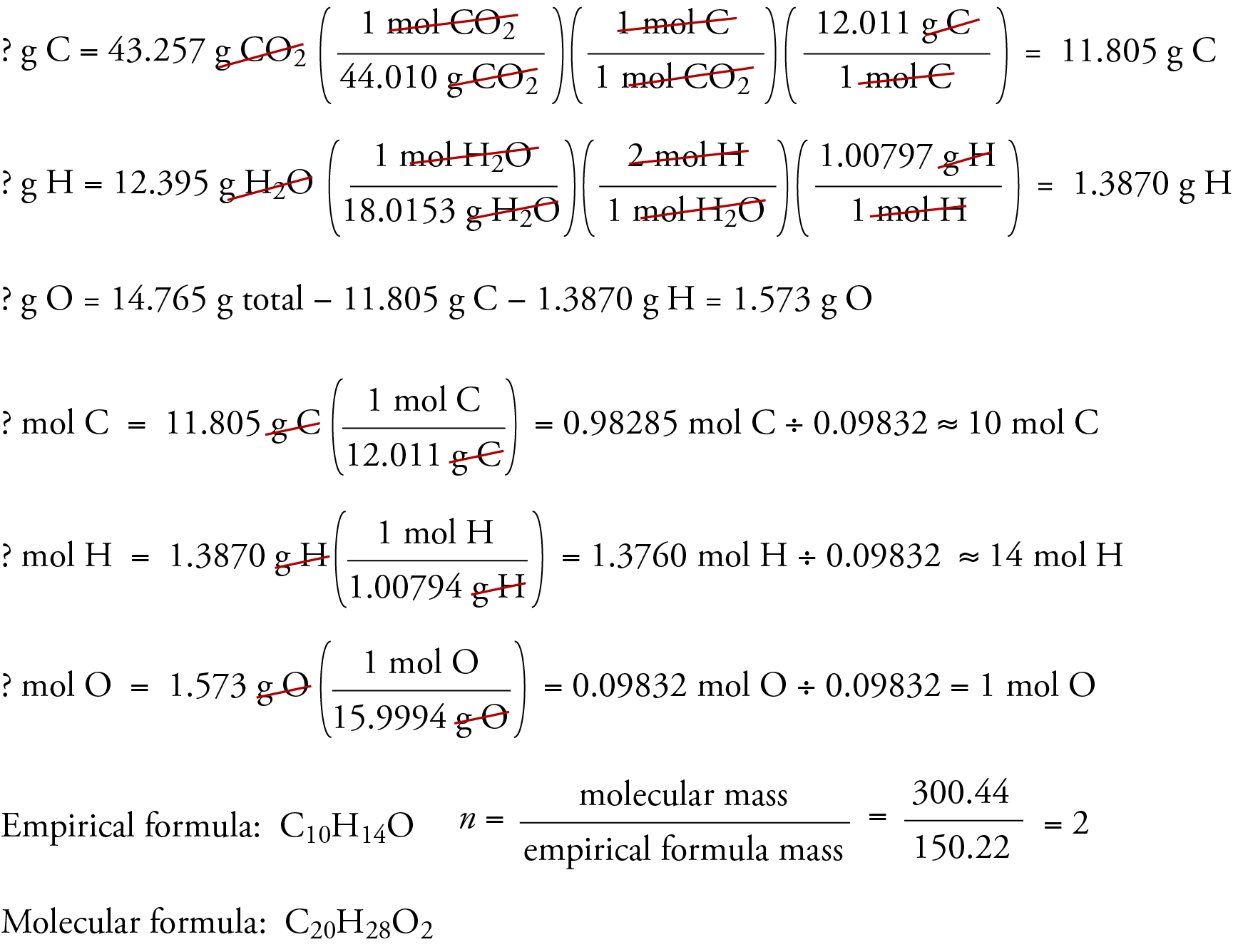
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

Bisphenol A, molecular formula: C 15 H 16 O 2, molar mass is 228.29 g/mol. | Download Scientific Diagram
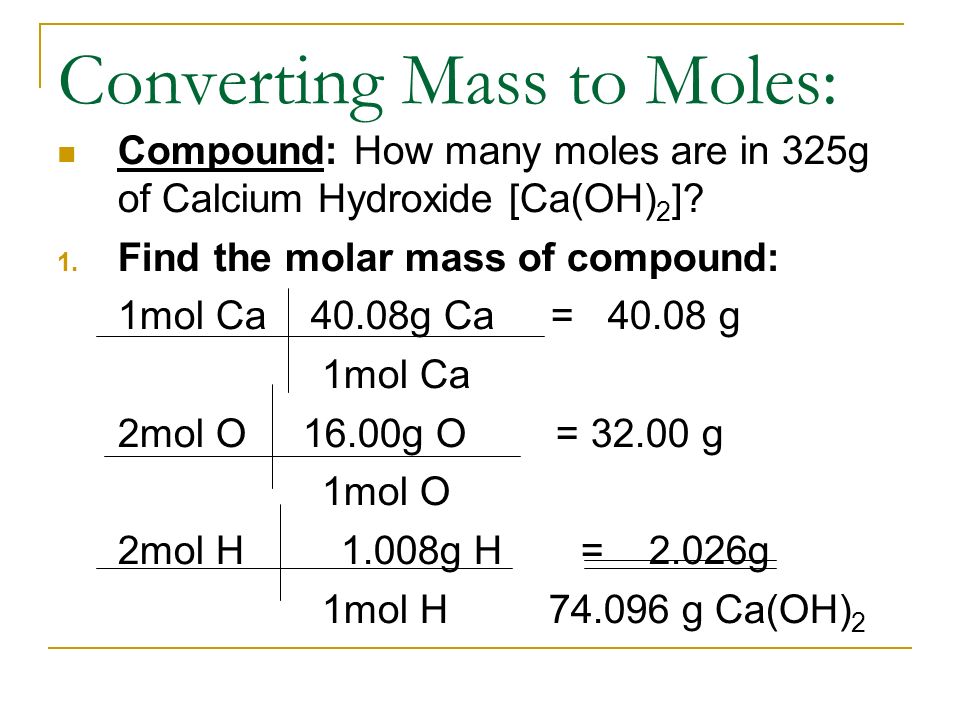
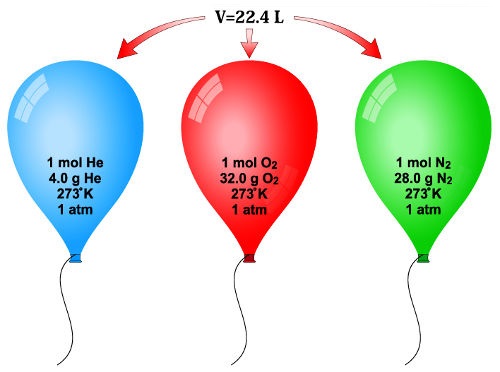
Chapter 11 : Matter Notes. Mole (mol) is equal to 6.02x10 23 The mole was named in honor of Amedeo Avogadro. He determined the volume of one mole of gas. - ppt download
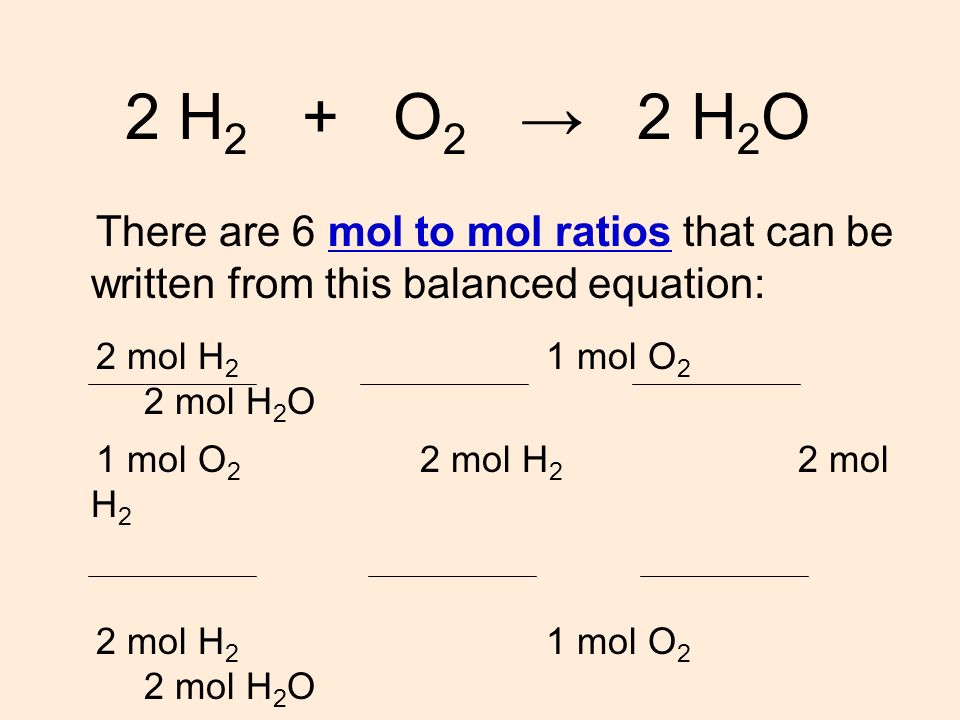
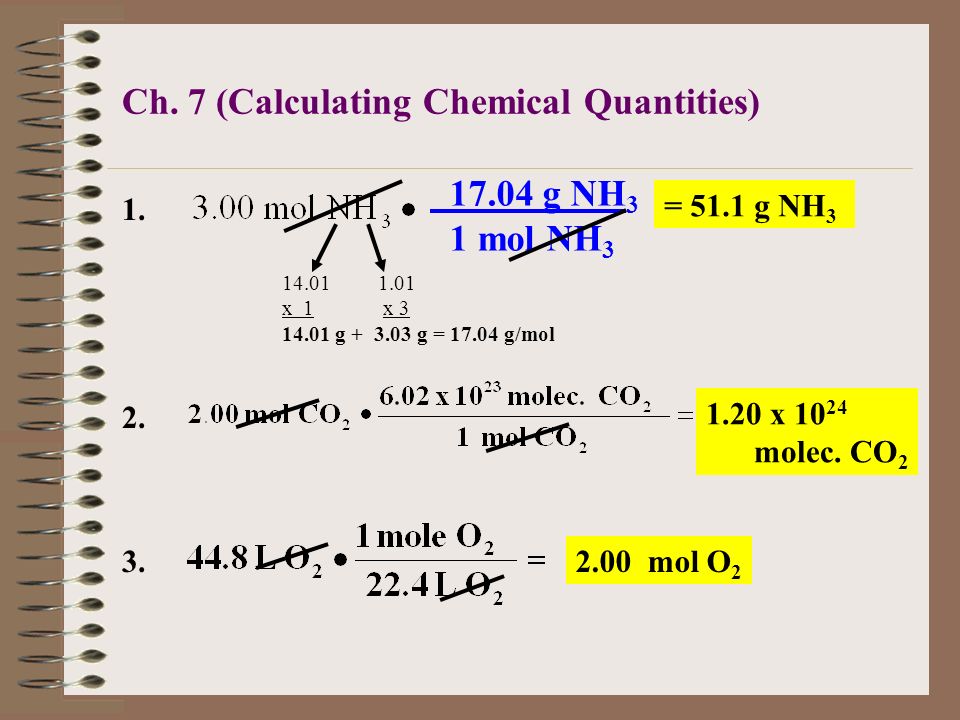
Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download

SOLVED: How many of each the following contained in 100 grams of CO2 (m=44.01)? calculate mol of C,O and O2