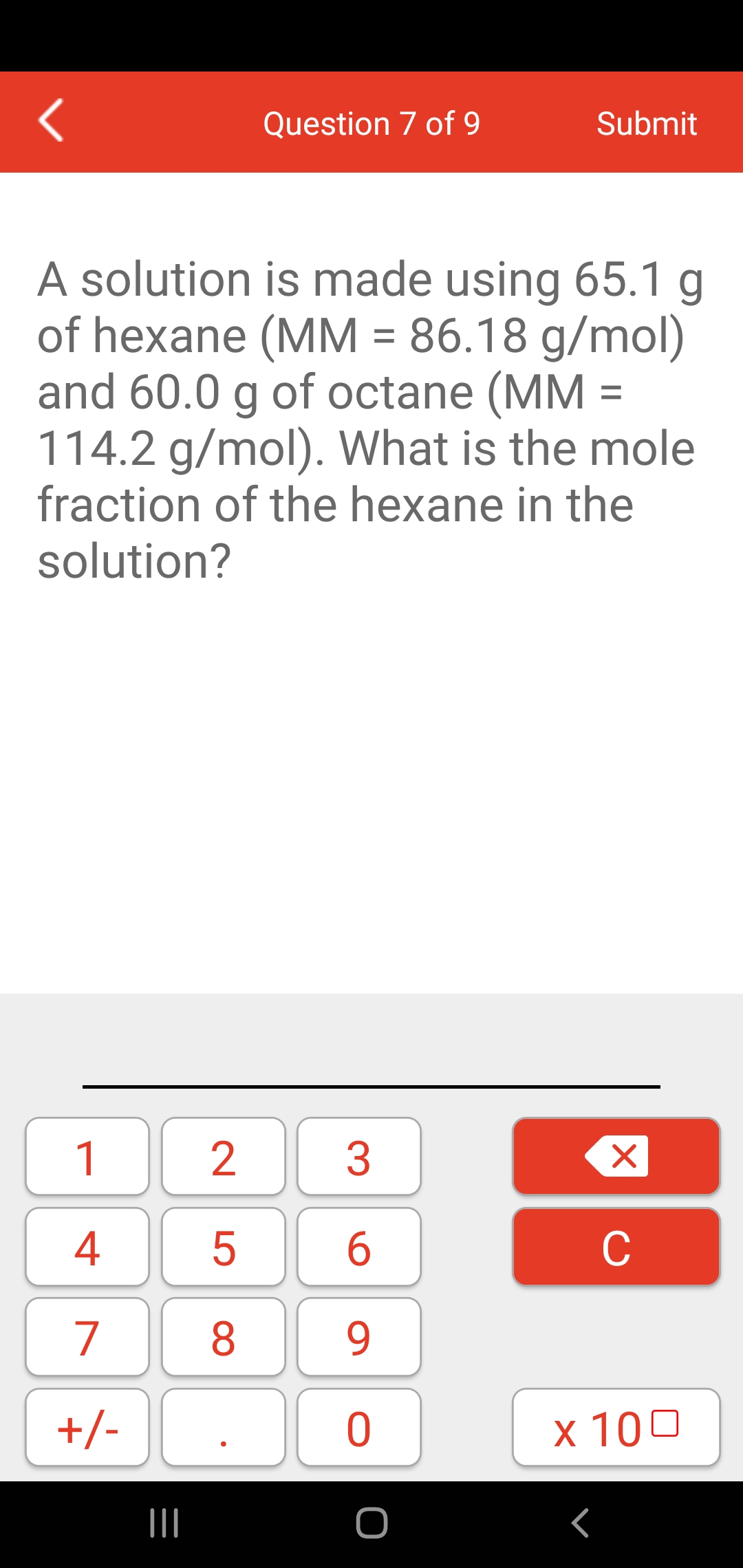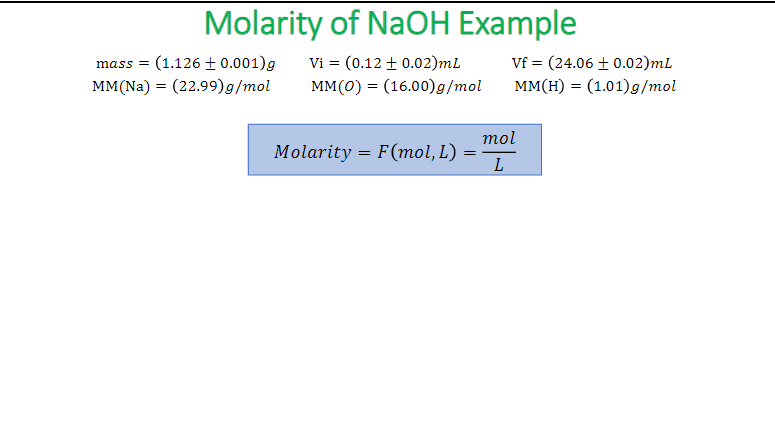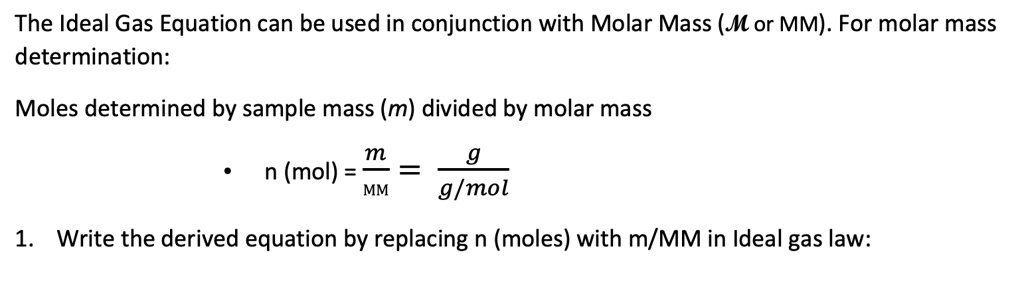
SOLVED: The Ideal Gas Equation can be used in conjunction with Molar Mass ( MM or MM): For molar mass determination: Moles determined by sample mass (m) divided by molar mass m (mol)

Bahco Wrench 9029C BH9029C Adjustable MOL Central Gran AP Wrench, Silver/Black, 6 Inch, 32 mm - - Amazon.com

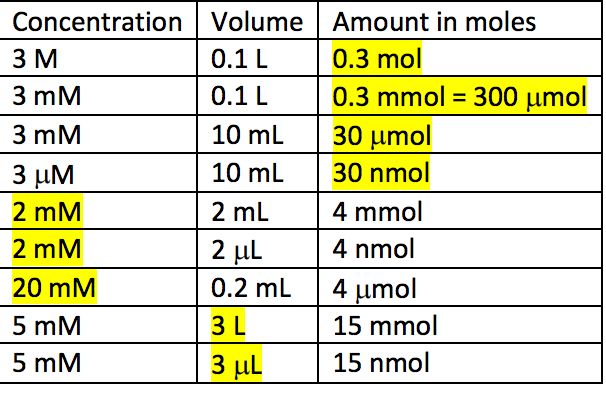
Chapter 5 A Matter of Concentration. Ionic Phenomena = Things that happen to ions, which can be observed. - ppt download
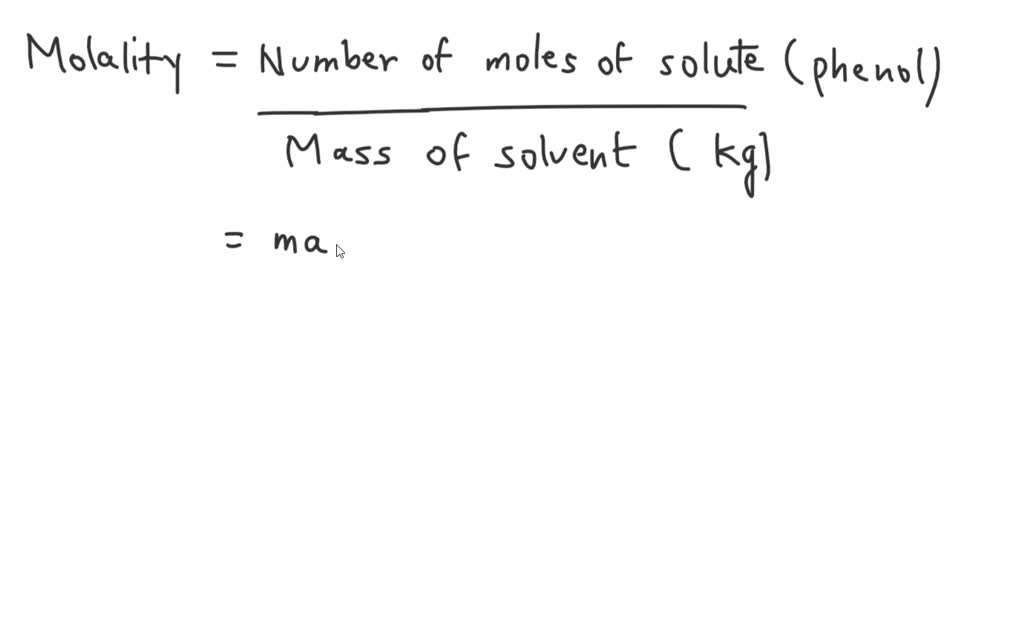
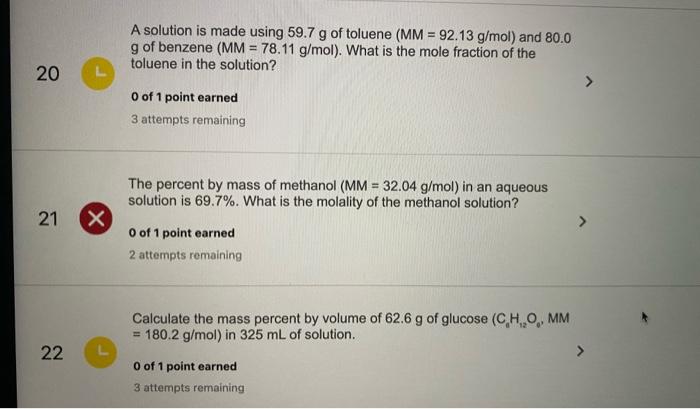
SOLVED: A solution is made using 79.9 g of phenol (MM = 94.11 g/mol) and 90.0 g of acetone (MM = 58.08 g/mol). What is the molality of the phenol in the solution?

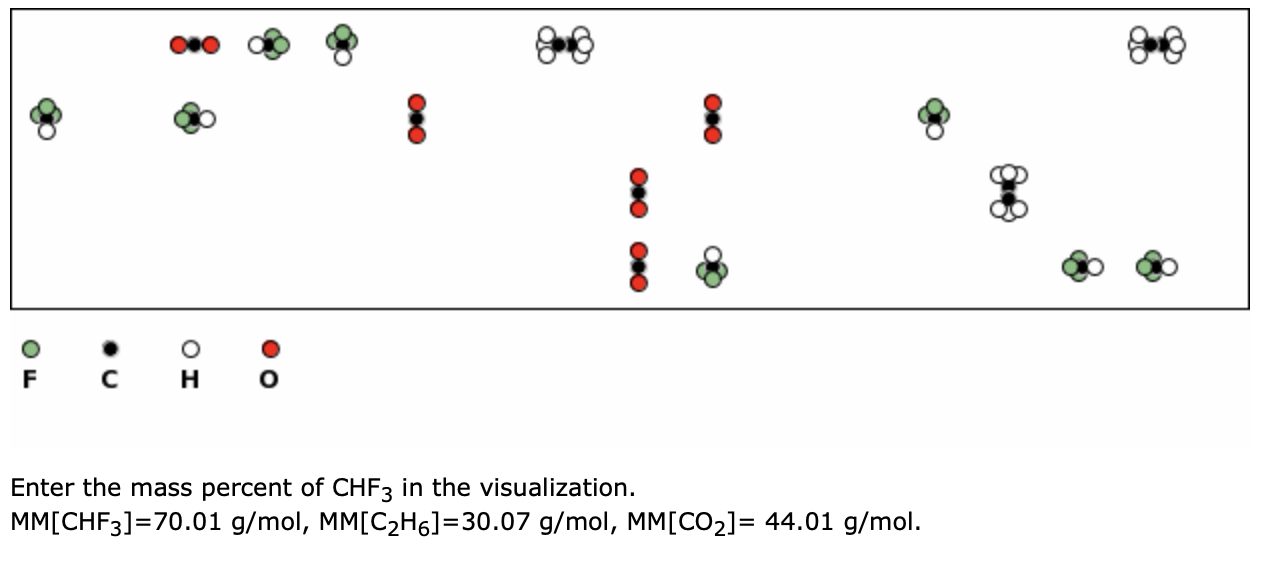
SOLVED: Question 21 ot 27 Submit What is the concentration in molarity of a solution which is 2.95 %mgv acetone (MM = 58.08 g/mol in ethanol (MM 46.07 g/mol)? x10

Molecular Dynamics and QM/MM Calculations Predict the Substrate-Induced Gating of Cytochrome P450 BM3 and the Regio- and Stereoselectivity of Fatty Acid Hydroxylation | Journal of the American Chemical Society

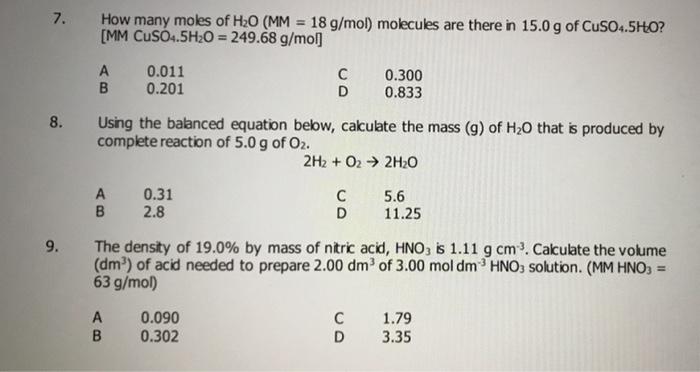
PLEASE HELP QUICKLY!!! MM H2O2 = 34.02 g/mol MM H2O = 18.02 g/mol MM O2 = 32 g/mol 2H2O2 —> 2H2O + - Brainly.com