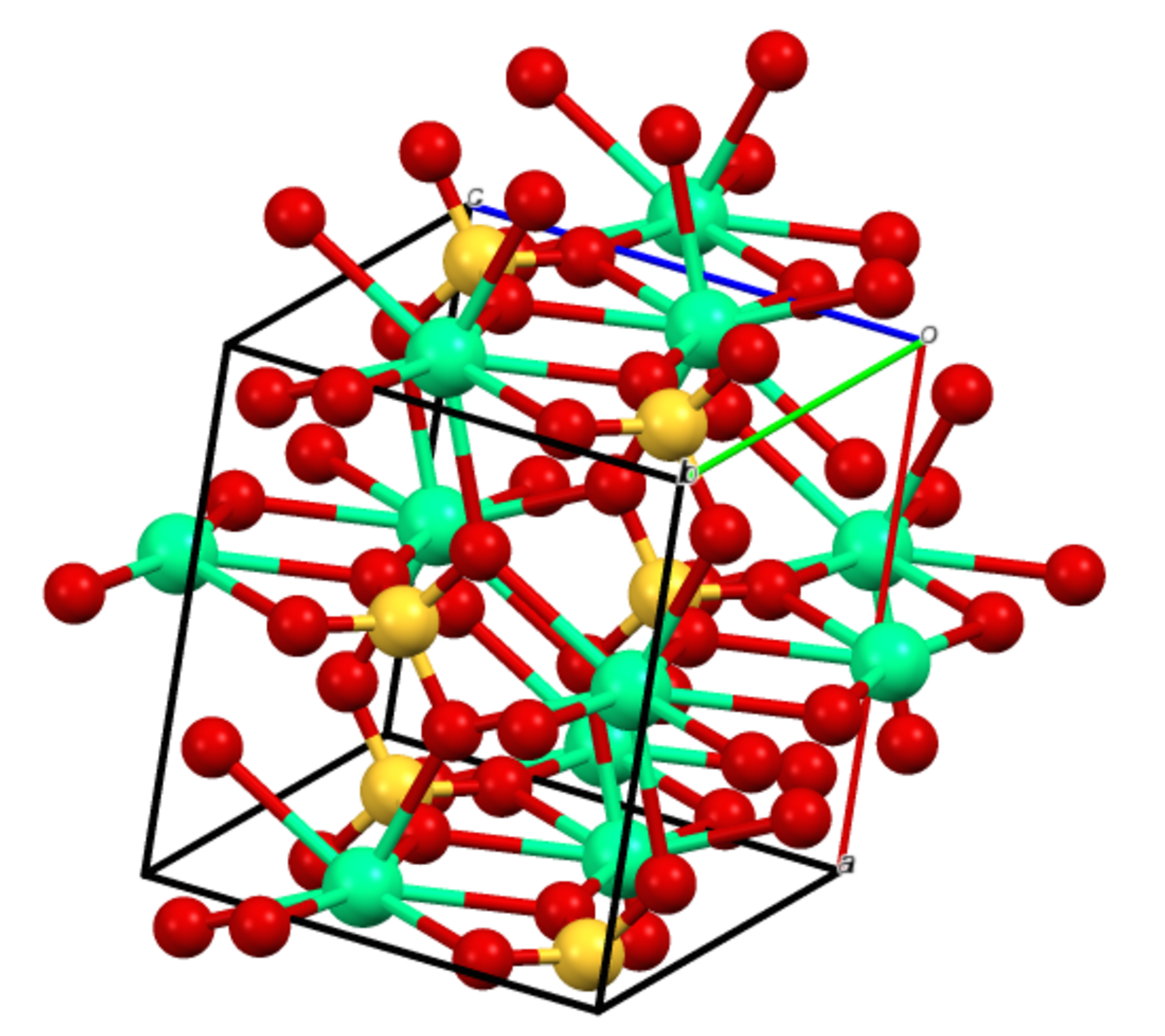
Gypsum (CaSO4.2H2O) mineral, crystal structure. Used for the production of gypsum board, plaster and in fertilizer compositions Stock Photo - Alamy

Thermodynamic Modeling of Calcium Sulfate Hydrates in the CaSO4–H2O System from 273.15 to 473.15 K with Extension to 548.15 K | Journal of Chemical & Engineering Data
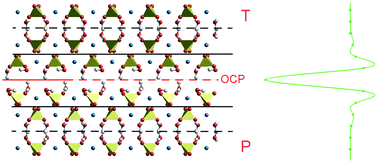
Interfaces structure and stress of gypsum (CaSO4·2H2O) penetration twins - CrystEngComm (RSC Publishing)

Gypsum (CaSO4.2H2O) Mineral, Crystal Structure. Gypsum Is Used For The Production Of Gypsum Board, Plaster And In Fertilizer Compositions. Stock Photo, Picture And Royalty Free Image. Image 17236747.

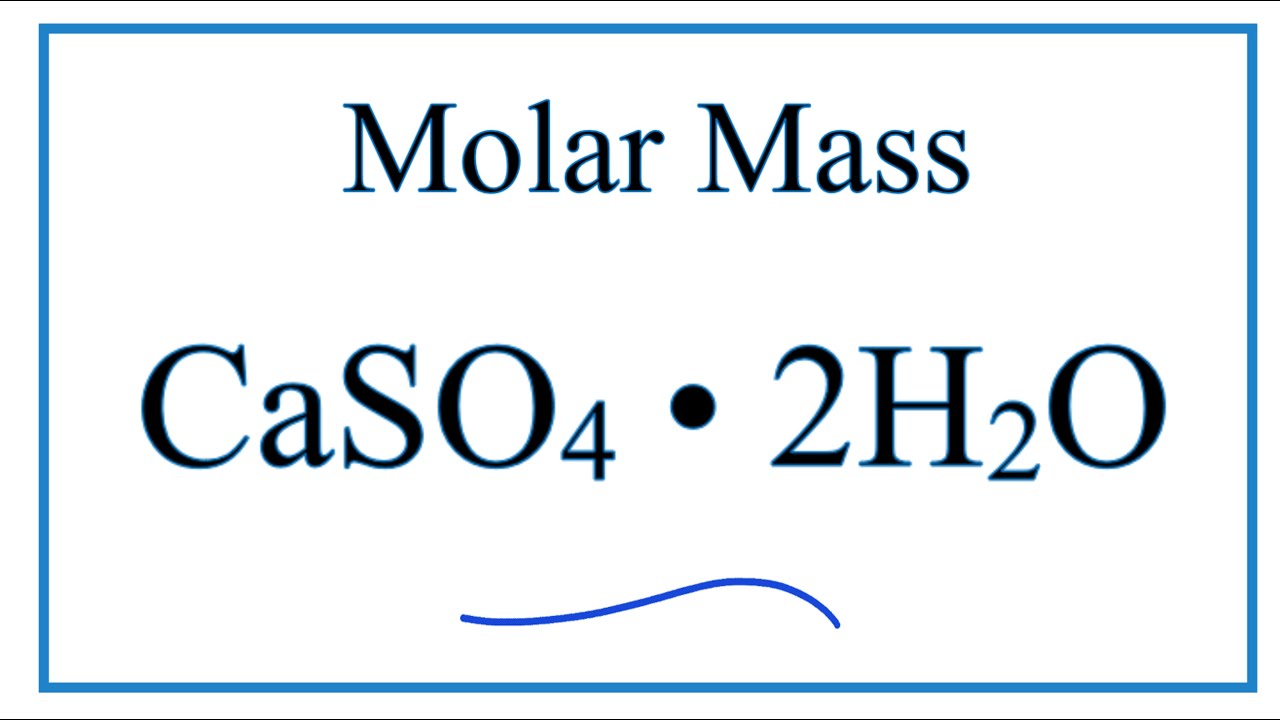
SOLVED: QUESTiON 5 In the following reaction CaSO4*2HzO(s) CaSO4(s) + H2O(g) the anhydrate is CaSOa(s) HzO(g) CaSO4 2H2O(s) none of these

Arsenic speciation in synthetic gypsum (CaSO4·2H2O): A synchrotron XAS, single-crystal EPR, and pulsed ENDOR study - ScienceDirect




![Y []Δ,205^∘C CaSO4.2H2O []Δ,120^∘C X . X and Y are respectively . Y []Δ,205^∘C CaSO4.2H2O []Δ,120^∘C X . X and Y are respectively .](https://dwes9vv9u0550.cloudfront.net/images/4281429/89baf354-9f4e-4244-93da-acebcca3fce9.jpg)