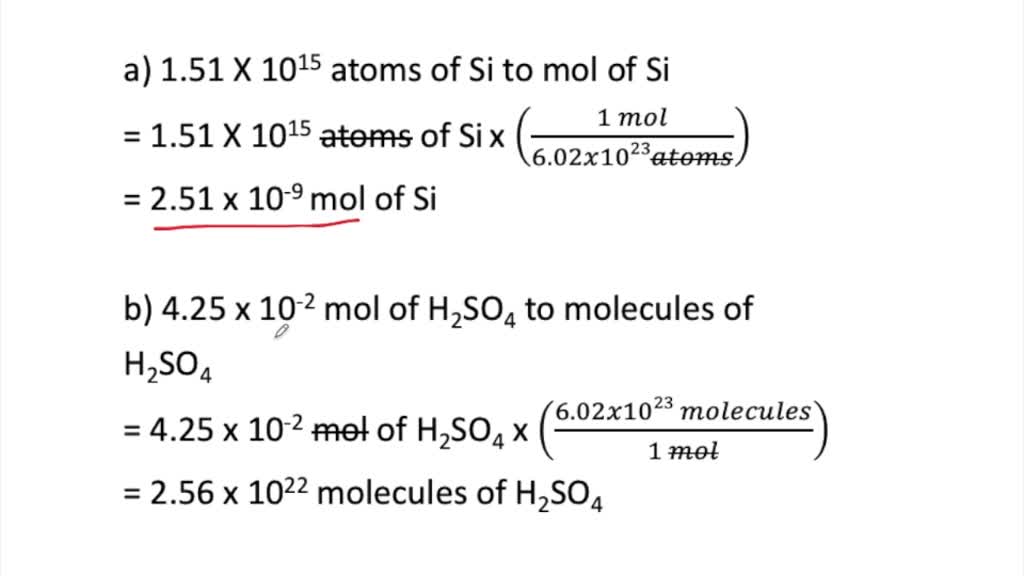
SOLVED:Perform the following conversions. a. 1.51 ×10^15 atoms of Si to mol of Si b. 4.25 ×10^-2 mol of H2 SO4 to molecules of H2 SO4 c. 8.95 ×10^25 molecules of CCl4

15 moles of H2 and 5.2 moles of I2 are mixed and allowed to attain equilibrium at 500 ^o C. At equilibrium, the concentration of HI is found to be 10 moles.

15 moles of N2 is mixed with 20 moles of H2 in an 8 litre vessel. 5.6 moles of ammonia is formed Calculate Kc for the equation, N2(g) +3 H2(g)= 2NH3(g) "+ heat"
15 moles of H2 and 5.2 moles of I2 are mixed and then allowed too attain equilibrium at 500^°C .At equilibrium the concentration of HI its found to be 10 moles .